Strategic Laboratory Management
Delivering total control in the physical and digital life of critical assets.
This is the Computype vision. We see a future where critical assets benefit from the most enabling identity – in physical and digital form – over the asset’s lifetime.
Our role is to maximize the integrity and functionality of asset IDs, to improve how work flows in the laboratory environment, and to give key decision makers the insights need to optimize lab performance moving forward.
Pre-scientific preparation: Logic demands a holistic solution
Whatever the assets – be they high value compounds, human biological samples, blood-derived products – reliable physical and digital tracking are key to maintaining efficient workflows.
Computype is ready to deliver the efficiencies and controls you need to optimize all lab data organization and management, by giving you an all-encompassing ecosystem of item ID products, systems and expert services.
Our goal is to make the most effective Digitally Connected Laboratory a reality for labs across the world.

1. Preserving laboratory integrity: The key is in the prep.
Whatever the task or tasks may be in any given lab, thorough preparation before the scientific work commences is obviously a fundamental priority. To gain robust results from the science, first everything must be in place to support an efficient, transparent and easily managed workflow.
At Computype we understand the crucial nature of pre-scientific preparation. We have perfected a comprehensive suite of products and services to make the process as straightforward and cost-efficient as possible.
For many organizations across the world, we’re the trusted partners who ensure the ID, tracking and managing of samples is totally dependable, and we’re the team with the knowhow and resources to facilitate data-driven laboratories.
The foundation of a data-driven laboratory
With the most robust pre-scientific preparation measures, data integrity for each asset is dramatically improved. It’s also key to supporting a genuinely effective Laboratory Information System (LIMS).
When a lab is properly data-driven, oversight is maximized, safety becomes optimal, and resources can be used in the most productive ways.
Laboratory Informatics
Laboratory informatics refers to the specialized application of information technology to enable and enhance scientific processes and the delivery of laboratory information. Digital identification and item attributes lay the foundation for robust informatics to inform laboratories and research facilities.
Data Quality
Data quality refers to developing and implementing quality management techniques to ensure the data is fit to serve the specific context and unique needs in laboratory environments. Data quality dimensions can include Accuracy, Completeness, Timeliness, and Accessibility, among many others.

Ensure a comprehensive pre-scientific process, expertly handled
There’s plenty of work to do through preparation phase. Samples must be collected, identified, weighed, stored appropriately, tracked, monitored… Computype is ready to support the entire pre-scientific stage for just about any laboratory, whatever its configuration.
So for example, maybe you would benefit from pre-printed labels, or your own efficient print workstations for print-on-demand capability.
If print-on-demand is not necessary, Computype can prepare and deliver pre-identified labware. We are enabled to take on many labor-intensive tasks such as tare weighing, sorting, kitting and label application.
Our capabilities also include creating item-level tracking solutions, to provide 20/20 oversight and co-ordination of the entire pre-scientific process.

Key quality factors around preparation
Supporting automation
It’s essential that 2D barcodes and linear barcodes are correctly positioned on each and every piece of labware. The codes must also offer crystal-clear reads at speed on every pass, if automation functionality is to deliver its benefits without stoppages.
Storage requirements are part of the spec
An ID system must take account of the storage conditions assets are kept in. Factors such as temperature, moisture, solvents or long storage timescales should never impact on the label’s readability or its original weight.
Single-source labware prepping is the ideal
It makes sense to use one supplier for all labware prep, as this guarantees consistent quality across all barcoding, tare weighing and ID longevity.
Continuity of supply
A supplier such as Computype has the global scale to ensure high quality labware, preparation services and expert process consultancy are always ready and available to support your activities.
Optimize use of your skilled staff
Preparatory activities such as tare weighing every plate, tube or vial and labeling are crucial – so rather than cut corners, many labs use skilled scientific staff to perform these laborious tasks. But in doing so these people are diverted away from their core scientific roles – essentially they’re prevented from doing the more productive work which they are trained and paid to do.
Computype is fully equipped to take responsibility for these administrative ‘house-keeping’ activities. It’s a cost-effective choice many labs make, because labware arrives perfectly pre-tare-weighed and labeled, instantly ready for use by skilled individuals in their important work. Just as importantly, those labels are designed to withstand the rigors of your processes, in precisely the right position to be scan-read without fail.
Integrating disparate lab systems and workflows
Our expertise can be particularly helpful when two or more labs have to align their respective systems and practices – through mergers and acquisitions for instance, or when collaborative projects are undertaken between organizations.
Proven, reliable sample identification is key, particularly where high throughput speeds and high volumes of assets feature – accuracy and speed have to be dependable.

Maintaining the most robust chain of custody
Clearly there are safety and legal implications in cases where you’re dealing with hazardous, therapeutic or controlled substances. In these circumstances it’s doubly important that the chain of custody delivers a dependable audit trail, no matter the scale or complexity of your processes. Computype’s long association with labs in life sciences, research and clinical disciplines helps ensure the integrity of the chain of custody.
Get the labeling right and the rest can follow
As previously mentioned, an automated tracking system is only as good as the labels at its heart. The nature of different storage and lab processes can impact on the readability of an item’s ID, so it’s imperative to utilize proven labeling specifically designed for certain conditions
Start tracking your success today.
Contact us now if you would like to learn more about Computype’s preparation products and services – our experts would be pleased to talk through all your options.
Schedule your Free Consultation
2. Data carriers: The digital life of an asset.
At Computype we believe that details for any asset within a lab or library should be quickly accessible in digital form. This is why we’re so committed to the ‘digital life of an asset’ concept – putting in place all the necessary hardware and software to enable asset tracking and the accruing of data related to each item.
Samples, specimens and critical assets can be immensely valuable in monetary terms. They may be the key to a transformational health advance, or a significant new industrial process innovation. But when assets inhabit complex systems at scale, and when development of such systems has maybe been less than well planned, problems can occur.
We know from experience that a holistic approach to creating an ‘ecosystem’ which supports comprehensive tracking and data collection is the ideal. Whether the assets are chemical, geological, or biological, the principle of creating a coherent and robust ID and tracking system applies.
Clearly this is important for supporting efficient workflows – and it’s central to delivering optimal productivity. In many scenarios, regulatory compliance, safety and legal requirements will also add imperatives for maintaining robust ID and tracking.
|

|
Choosing appropriate digital ID for your operations
So we come to the physical identifier itself – a label, tag, inlay or some other form of marking. Barcodes continue to prove their worth in tracking systems. They can be relied on to deliver consistent ID performance through high speed processing and where high volumes of items need to handled without interruptions to the workflow.
There are several types of barcodes that can be used – 1D/linear, 2D or stacked. Symbologies can be discrete or continuous, and both have different advantages to suit different applications.
Computype’s deep expertise in barcoding for the broad range of assets used in scientific applications means we can recommend the right symbology for your application.
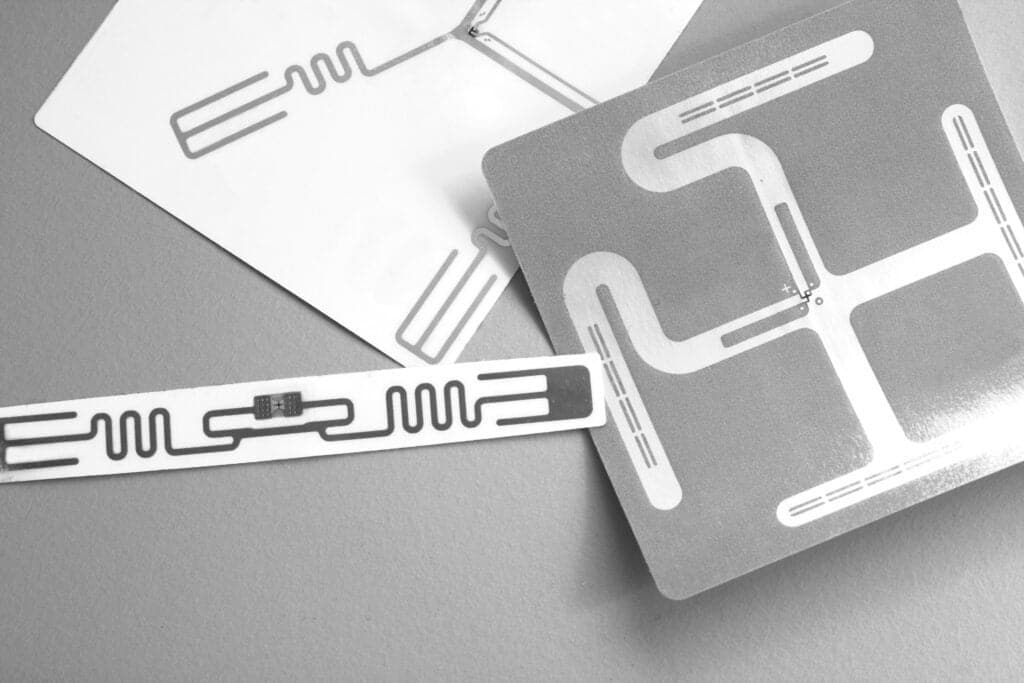
RFID inlays are another ID useful option. These can be sensed and read using near field communication technology, and have the potential to hold more information than is usually the case with conventional labels. Providing data such as expiration dates, remaining life, number of tests performed, or verifying the authenticity of a reagent or consumable – RFID can perform a number of helpful functions.
As the name suggests, hybrid labels are those that are enhanced by the addition QR codes, electronic article surveillance tags or RFID tags. These labels add more functionality and data carrying abilities to the ID.
Delivering sequence integrity and just the right information
Sequential IDs lie at the heart of a strong tracking system. Although in some cases a sequencing system may be based on the OEM instrument’s default numbering convention, this may not be the most suitable basis to work from for your organization. A custom arrangement can add higher levels of efficacy and relevance to how particular processes operate. A prime concern is sequence integrity, and a custom solution can guarantee this.
Track item attributes with digital IDs and robust traceability
Clearly the basic inclusions will include significant dates and times – origination and processing events, for instance. Location, project identifier, lab details will also have to feature. It may be that variable data needs to be associated with each ID – such as patient information. The IDs will also link with lab notebooks and laboratory information management systems (LIMS).
Laboratory Barcodes
The barcode optimized laboratory
Barcodes have been the mainstay of asset ID and tracking in laboratories of all kinds for decades. They remain the ubiquitous data carrier of choice across the globe because essentially, barcodes in all their guises are so very reliable, economic, flexible in the tasks they can handle, and a natural fit with automated systems.
A barcode-based lab system combines high speed data collection with exceptional accuracy – barcodes can be read in a fraction of a second, with a typical error rate of one error per 10 million characters. And as a well-established technology, the best barcode systems are both thoroughly proven and economic to adopt.
What do you need from your barcodes?
There are plenty of things a barcode can do for your lab, and your own requirements will likely be very specific. The following are general considerations and possibilities, so it makes sense to seek specialist advice to discover the solution that’s fully tailored to your lab’s needs.
Factors influencing barcode label choices – these can include:
Container shapes and sizes
Labware shapes and sizes may impact labeling options. Make efficient use of space with 2D barcodes on caps, closures, and bottoms for additional information. Sourcing low volumes of pre-barcoded labware in irregular shapes or sizes can often be costly through labware manufacturers, so be sure to discuss your needs with a trusted partner.
Environment and testing requirements
Laboratories are often harsh environments, exposing samples and containers to chemicals, temperatures, abrasion, and other necessary procedures. Barcodes need to survive that environment without fading, failing, or falling off.
Any regulations affecting the sample to be labeled
Many samples and testing procedures are subject to regulations regarding proper tracking and handling. Make sure your identification and tracking partner is aware of the samples and specimens your lab manages.
Adding multiple codes to a single container
Consider adding multiple codes to your labware for additional information. For example, a linear barcode along the side of your tube could pair with a datamatrix code on the cap or bottom. This could allow you to track additional information for your facility or for your customer.
Common inclusions in barcode data are a unique identifier and a prefix which features your facility’s code. This can also be presented in human-readable form. Other data that can be ‘barcoded’ include attributes such as collection date, but keep in mind that barcodes have a lower data capacity compared to RFID.
Don’t crowd your codes
Human-readable information printed on the label can provide a simple ID failsafe. If a barcode or tag becomes unreadable for some reason, text can still identify the asset.
Be careful not to crowd the label with too much text. If you don’t need the information to take manual action with the container, leave it off.

Barcode sequence management: getting everything in order
Many labs and life sciences facilities entrust their labeling to a provider like Computype. In part, this option is taken in order to avoid the labeling demands and potential issues already covered in this text, but achieving robust sequence management is also a major factor in the decision to outsource.
Computype sequence management of labels and markings guarantees that no duplications can occur.
It’s a discipline we have dedicated many years and resources to, so that today especially in safety-critical scenarios such as blood transfusion medicine, our labels and their sequencing are the pre-eminent, go-to solution for organisations across the world.
In truth, any sample or specimen can be valuable for any number of reasons. They can represent considerable investment in time, money, research, labor hours and pure novel innovation, so ensuring label sequence integrity always matters.

A system proven in safety-critical practice
Computype Sequence Management Services center on a database that allows tracking of sequential numbers printed on labels. The example of transfusion medicine demonstrates how our services extend throughout a system to deliver dependable ID and tracking of thousands of items, often across multiple locations.
Clearly, donated blood must be perfectly and accurately tracked to ensure it can be safely utilized when needed. An unidentifiable blood bag is essentially a wasted donation as its provenance is lost. Through robust sequence management, sequences are traceable by part number, individual organization, or transfusion medicine processing center in cases of multiple locations. If a center has multiple locations, we can group all locations together and track the sequences to ensure no duplicate numbers.
To avoid duplication, Computype’s vision-based system and digital offset printing actions verification of several key factors:
- Every symbol on every label is automatically scanned multiple times
- Each decoded number is within the range of numbers requested and is not duplicated
- All symbols are in the correct position
- The symbols are scannable and decode accurately
- The are no gaps in the sequence
As an added failsafe, Computype will subsequently store sequences for customers to make sure duplicates are never created, even from an old order.
The where and how of label placement
Correct and consistent placement of labels is crucial to maintaining scanning effectiveness, and in the case of automated scanning, correctly affixed and positioned labels are absolutely essential. Manual application cannot achieve the necessary accuracy and may also cause wrinkles in the label – a sure-fire recipe for scanning failure.
Linear barcodes require a clear line of sight and an optimal scan angle, especially when passing by fixed scanners. Precision label designs are vital to ensure successful, dependable scanning.
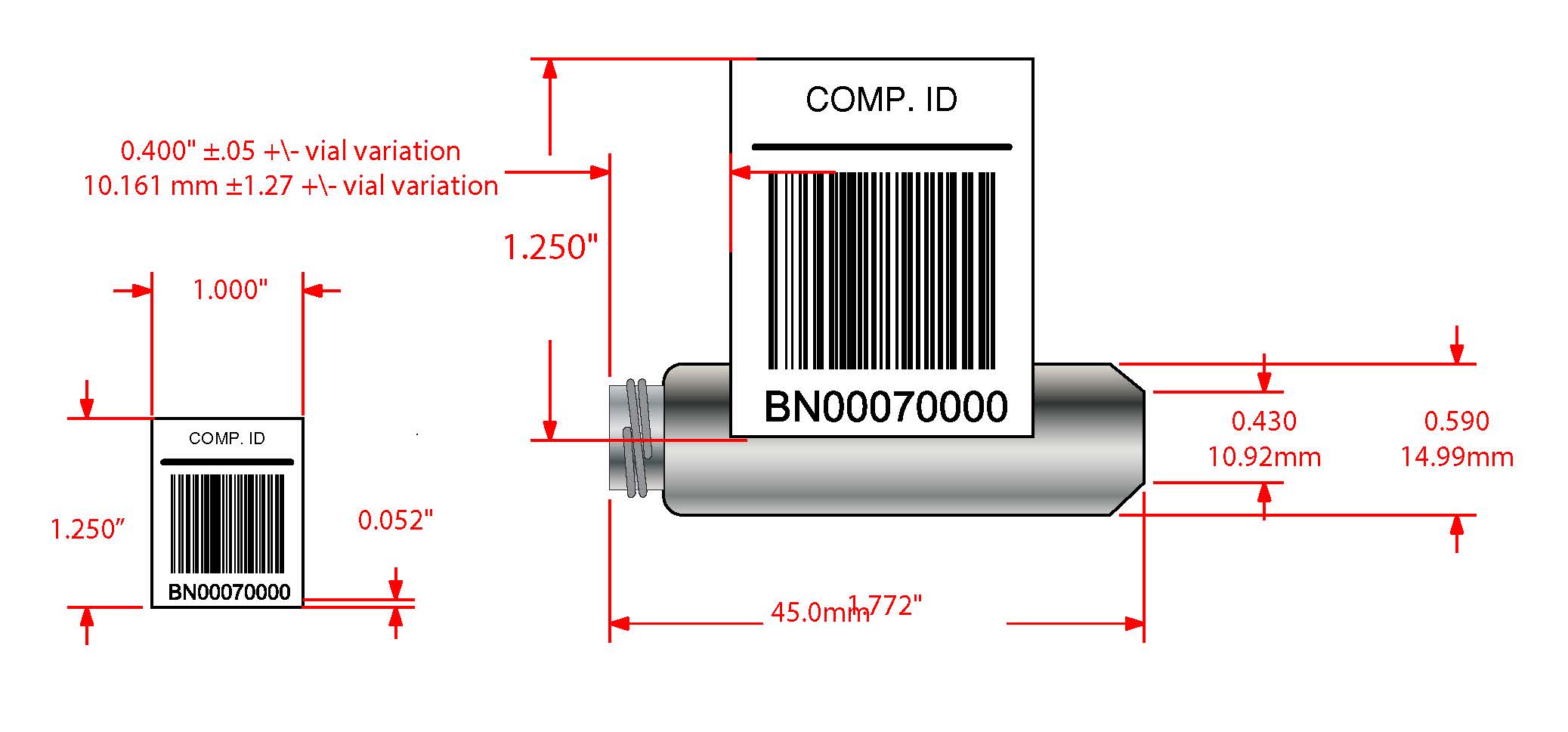
Custom barcode scanning example for diagnostic instrumentation
When designing a barcode-optimized laboratory, ensuring successful scans is a critical requirement. Our team had a rewarding opportunity to develop a custom solution for a global industry leader in diagnostic instrumentation. Laboratories and physicians utilize testing equipment sold by this company to test samples for pathogens.
This customer was experiencing friction in their sample processing workflow due to multiple failed scans from a single-line scanner. Single-line scanners only scan one line through each barcode, and any minuscule defects may result in a failed scan. Ultimately, these failed scans will compromise the integrity of the sample.
The laboratory testing equipment designed and sold by this customer is required to process samples automatically, including overnight, without supervision. Unfortunately, in cases of failed scans, the equipment would stop until an operator could intervene and address the failed scan. Therefore, in cases where an operator would load samples for overnight processing, they could encounter a significant backlog of unprocessed samples the next day.
Our team responded by developing new technology for high-speed scanning that would target barcode defects. This system scans each code, which are 3/10 of an inch in height, upwards of 50 times. Specific parameters are then set to identify the maximum number of failed scans allowed per code. If more scans than allowed were to fail, the barcode would be scrapped. This process ensures that unreadable barcodes will never halt a sample processing workflow.
Ultimately, Computype created a work center within our manufacturing facility dedicated to this particular customer and explicitly designed to quality check the barcode labels to this stringent specification. This system allowed us to improve service to all of our customers moving forward.
Need more information on RFID?
Laboratory RFID solutions are complex, but in the right setting they can yield significant results. You can schedule a free, no-obligation consultation with one of our experts to learn more about what RFID could look like in your lab.
Develop a Strategy for Your Laboratory
Ready to get started tracking success in your lab? Speak with one of our identification and tracking experts today. Get Started Today
At a glance: laboratory barcodes vs RFID
Barcodes |
RFID |
Scan only one item at a time |
Scan multiple items at once |
Slower read speed |
Faster read speed |
Read-only, data cannot be changed |
WMRM: Write Many, Read Many |
Requires line of sight |
No line of sight required |
Less expensive |
More expensive |
Easier to implement |
Expert integration required |
Identify item type |
Uniquely identify each item |
Lower data capacity |
Higher data capacity |
Consistent and reliable |
Metal and liquid can impact signal |
Fewer security opportunities |
Allows for greater security |
Laboratory RFID Solutions
RFID smart labels for more performant specimen tracking
Today it’s common for specimens to be shipped between several labs and facilities for various processes to be completed, and this can create issues for dependable sample ID and tracking. RFID labeling – particularly when scanned by smart phone – makes for a simple and convenient method of checking the ID and process data relating to the sample.
It’s also possible to equip a shipping doorway with sensors which can detect individual samples as they enter or leave a facility – even when the shipment includes many specimens – without the packaging having to be opened. It’s this kind of affordable functionality which makes RFID a valuable tool for reducing errors while increasing throughput, and lowering labor costs.

RFID applications in diagnostics
In-vitro diagnostics is an expanding discipline – molecular diagnostics, next-generation DNA sequencing, tissue diagnostics, clinical diagnostics, companion diagnostics, cellular immunotherapy and a host of new point-of-care processes are pushing medical boundaries in many different ways.
At the heart of these advances lies the specimen. Whether it’s blood, saliva, tissue or some other biological sample, the specimen has to be readily identifiable at every stage of a process over its lifetime.
RFID-enabled identifiers can authenticate proprietary reagents and other consumables, thus the chances of counterfeiting are eliminated. RFID also informs predictive maintenance – technicians can be alerted when an important consumable is becoming due for replacement.
You can also store quality indicators in user memory – manufacturing date codes, or a ‘diary’ of process steps completed for example. And of course, such data can be read and stored in external databases, including cloud-based. As an added failsafe, information can also be held within barcodes and in human-readable form.
And the power of RFID to hold data in various ways in a fully automated diagnostics process means the probability of human error can be reduced to very low levels.
Automated diagnostic instruments
From their inception in the 1980s, automated instruments began to perform some tasks which had previously been handled manually. The technology steadily grew from this position of semi-automation – often driven by the twin goals of freeing skilled staff to perform more exacting tasks, and the standardization processes for quality and consistency of approach.
RFID joined the diagnostics mix and made it possible to control the use of proprietary consumables and to authenticate reagents before processing. This also led to the use of RFID in tracking high volume consumables.
Today many of the instruments engaged in in-vitro diagnostics essentially constitute fully enclosed production plants, where specimens pass through automated process stages with no human involvement. Over time, barcodes have been supplemented by RFID identifiers, so that now, information can be stored in the integrated circuit chip carried by the consumable itself. This data can be invaluable in maintaining quality control.
Naturally, as RFID’s application grew across many sectors of science, technology and industry, the cost of RFID transponders became lower. Now they’re widely used in table-top automation systems, and are a perfect fit for contemporary diagnostic laboratories.

RFID for authentication
With many reagents being designed specifically for use in certain automated instruments, it’s important to ensure that unauthorized products never enter the system. RFID can be used to halt operation if inappropriate reagents are detected. It can also disable a chip when tampering is detected, so the offending cartridge can never be used.
RFID in predictive maintenance
RFID transponders are increasingly integral to the smooth running of many automated instruments and systems. They now offer powerful memories and a range of new functionalities, which are contributing to more efficiencies and predictive maintenance improvements. Maintenance programs can typically encompass everything from monitoring reagent levels to component wear, firmware upgrades and software modifications – RFID can help maintenance personnel stay on top of all such requirements, and to reduce the risk of expensive breakdowns and process downtime.
RFID for quality control and automated data logging
It’s possible to write information to an RFID IC chip as a process event occurs for a given sample. As a result, data such as the date and time certain actions were performed can be logged, along with information on weight, environmental parameters or cycle times. It can alert for process variables outside acceptable limits, and highlight a need for corrective action. All this functionality contributes to heightened quality control, alongside the overall continuity of processes.
RFID is now enabling low cost data logging. The sensors can either be passive or incorporate their own power source to drive the chip. Lab technicians now have a way to record data relating to the sample directly, with updates actioned at regular intervals. This is particularly useful in the case of specimens which must be held withing defined temperature limits. The RFID chip can be programmed to only record temperature deviations outside the prescribed limits, with the data readable to a mobile phone.

RFID for finding misplaced or shared assets
Many portable assets such as analyzers, pipettes, reagents, or even specimen slides are shared between workgroups. Typical tracking within labs or work areas for these items often consist of emails, spreadsheets, or even post-it notes. Tracking them down can waste valuable time. Instead, commonly shared assets can be equipped with RFID tags and a handheld device can be used to find them by simply waving it in an area until a signal is received.
Store large amounts of sample data with RFID example
Tracking large amounts of data is particularly difficult in laboratory environments due to the limited surface area typically available on most sample containers. In one real-world example, our team had the opportunity to collaborate with a company providing hardware and consumables to laboratories to enhance their offering.
Maintaining successful read performance is one of the primary issues when labeling an asset with a limited surface area, as the small label can only accommodate a small antenna. The customer was building instruments designed for pre-spotted microplates in this particular use case. Pre-spotted well plates need to identify each well individually, and many laboratories struggle to accomplish this accurately and reliably.
By adding RFID tags to the plates, and readers to the instrument, the data contained in the tag would be picked up by the instrument upon insertion of the plate. Each tag would include a data map of the associated pre-spotted plate, allowing the instrument to auto-populate the map and verify plate authenticity simultaneously.
The ultimate result was a highly customized RFID label that complimented their instrument platform. The RFID labels met both the requirements of data transfer mapping and verifying authenticity, ensuring performance and competitive advantage.
3. Sample containers and specialities: The physical life of an asset.
The risk of mislabeled samples
Mislabeled or lost samples are likely going to cause problems. One failed pre-printed barcode label can create very serious health and safety issues, legal liabilities, expensive losses, interrupted workflows, disruptive downtime, unplanned-for labor hours, or risk of failing regulatory audits.
So well-considered preparation lies at the heart of sound risk management. The labeling strategy you choose has to take account of factors such as exposure to potentially degrading environments, processes, storage conditions and timeframes. Whether it’s vial labeling, microplate labels, cryogenic storage labels, or GHS compliant labels, these physical data carriers inform your digital traceability platform, so they must be perfectly suited to your workflow characteristics for the whole system to work.
Managing labware and equipment
All manner of laboratory assets and items can be marked and managed with the application of suitable barcodes. Samples and specimens are obvious candidates for ID and tracking, but chemicals, compounds, reagents and biologics can also be included. Freezers and other storage equipment, sequencers, general lab equipment and instruments, computers, laptops, tablets – when everything is identified, its management can be optimal.
Explore identification opportunities for your labware:
Labware procurement
Through our pre-barcoded labware services program we can source labware directly for our customers, and your containers are carefully sourced according to your desired specifications. Our partnerships and buying power give us more choices and quick access to equivalent labware from alternate suppliers if needed. We can also advise laboratories who wish to source their own labware to recommend the best solution to match process demands and budget.
Irregular shapes, uncommon sizes, low volumes
Our facility, team of experts, years of experience, and an unyielding dedication to supporting laboratories means that we are designed to meet custom and bespoke needs. When working with irregularly shaped labware, non-standard sizes, or low volumes, custom labels can become costly if purchased through a labware manufacturer. Our focus and our resources allow us to support the unique needs many laboratories face.
Specialty labeling for life sciences product manufacturing
Custom-engineered label materials and construction
Our labels are carefully constructed and expertly engineered to withstand the harshest environments and preserve your data and sample integrity. We’ll consider your lab’s environment, testing requirements, handling procedures, and sample type before designing your labels to make sure each aspect meets your needs.
Increase labware functionality
Labeling and marking labware can do more than identify samples and specimens. We encourage our customers to enhance their labware and increase its functionality by adding grad lines, color coding, write-on patches, logos, and human-readable text.
Self-laminating labels
Self-laminating labels offer an additional layer of protection during extreme testing procedures. Wrap-around labels for tubes and vials, as well as our FLAP labels for histology slides provide testing lab’s with a quick and easy way to preserve sample integrity. Please take a moment to learn more about our FLAP labels in the video below.
Advanced marking technologies
Labeling in-house comes with several limitations. In addition to the significant time and energy required, labeling in-house will typically mean using pressure-sensitive labels. The equipment and procedures required for more advanced marking technologies is simply not realistic to achieve within a laboratory.
Direct mark
Direct mark technology prints and cures specially formulated ink directly to the surface of your glass or plastic labware. This creates a near permanent mark capable of withstanding harsh chemical baths, extreme temperatures, and long-term storage.
The ability to print on to the surface of labware without a layer of media also makes it possible to include functional markings like grad-lines.
Laser
Laser marking technology is suitable for metal, plastic, and glass, and can print on both flat and curved surfaces. Laser marking provides strong chemical, temperature and abrasion resistance. Success with laser marking is heavily connected to the labware used, and need to be discussed with your labeling partner.
Ceramic
Ceramic IDs are permanent—guaranteed to survive as long as the container itself. Ceramic barcodes are made from a special clay that will permanently bond to a glass container when fired in a kiln. In addition to both linear and 2D barcodes, small round colored markers can be placed on the bottoms of glass vials.
This method is not compatible with plastic containers due to the high temperatures involved in the bonding process.
Fused
Bonded directly to the surface of your glass vials, fused labels are extremely durable, especially against chemical exposure and high temperatures. Fused barcodes have proven to withstand temperatures as high as 230 degrees Celsius, as well as cryogenic storage and heptane or IPA soaks.
Direct Mark barcode labels ensure dependable tracking to support research example
Computype is currently partnering with a healthcare company pursuing a new project within the biobanking segment that involves gathering over thirty-five million biological samples from more than one million participants. This project aims to track participants over months, years, and even decades in hopes that researchers will be able to discover new information about various diseases.
To proceed with the project, the company had to define a means of incorporating label automation into their process; with as many samples as they were planning to manage, electronic records were deemed necessary. Efficiency is also essential for a project like this; there is only a short time frame after surgery for tissue to be collected and preserved for later use. The challenges regarding this project include utilizing solutions that can correctly and reliably mark each sample most ethically and efficiently most ethically and efficiently.
With all of these criteria considered, the company discovered their need to find a solution that was proven to be more durable and precise than a standard pressure sensitive pressure-sensitive label. Additionally, standardized and proper care of samples is critical for any project that involves human tissue.
To protect samples from contamination, which can occur if chemical surface treatments are used on containers, Computype utilizes a unique method of direct mark technology that does not require surface treatment of the tubes. This method doesn’t damage the structure of containers either. This guarantees the ethical and standardized handling of samples throughout the entire process.
Additionally, Computype can adequately duplicate the existing 2D barcodes and produce a corresponding 1D human-readable mark on the outside of the tube to preserve the integrity of the samples. This is necessary because human-readable information and linear barcodes function as a redundant identification system. If the 2D barcode becomes damaged, two additional ways to identify the sample remain: either scan the linear barcode or read the human-readable information. This way, researchers can proactively follow the participants; for example, if in 10 years a participant develops a disease, researchers can quickly scan the barcode on the original sample and compare historical information attached to their tissue sample.
Using Computype direct mark technology, researchers are gaining the opportunity to start examining factors never seen before that cause diseases. We prioritize the integrity of our solutions in this space and will continue to provide reliable barcoding solutions to ensure
samples remain reliable for future research purposes
Start tracking your success today.
Contact us now if you would like to learn more about Computype’s preparation products and services – our experts would be pleased to talk through all your options.
Schedule your Free Consultation
4. Sample preparation: Ready-to-use labware.
When developing their identification and tracking strategies, multiple successful strategies are available for laboratories. Ultimately, our goal is to help design the right solution for each unique circumstance to support scientific growth and critical advancements in medicine. Whether your lab is best suited for an in-house labeling strategy or whether you may benefit from receiving fully prepared, pre-identified labware, there are multiple avenues to consider before making that final decision.
Laboratory Label Automation
Labels engineered for automation
The ‘nuts and bolts’ practicalities affecting your solution need to be carefully assessed. For example, at Computype one of our commonest customer enquiries relates to the ‘failing’ of label applicators in automated systems. Invariably the problem is down to unsuitable labels being used.
Something as simple as ensuring your labels are fully compatible with your applicator keeps the labels – and therefore your workflows – running smoothly. It’s a surefire way to avoid issues caused by adhesives jamming systems, or problems around liners being too thick to apply reliably.
If you do need to maintain labeling and printing in-house, it is critical to work with your labeling partner to ensure that your label materials are compatible with your printing and automation equipment. The wrong adhesive, wrong liner thickness, wrong flexibility, and even the best machine will jam. Material compatibility is key to in-house labeling success.

Keeping it as simple as possible
In many cases, a simple print and apply set-up can be all that’s required. It’s the easy fix that can be based around an entry-level, out-of-the-box applicator. Our options are selected to be affordable and intuitive to use, right off the bat.
Two of our most popular applicators give the ability to immediately improve label placement accuracy and application speed, with no expert set-up needed:
cab Axon 2
This applicator combines its impressive printing power and unique and user-friendly label application for a truly convenient, benchtop print-and-apply solution.
cab SQUIX + s3200
The cab SQUIX s3200 precisely prints and applies labels onto microplates in just 8 seconds for consistently reliable results for reduced waste and more efficient downstream processes.
cab Axon 1
The Axon 1 accommodates vertical application for capped or uncapped tubes, accommodates a larger range of sizes. Ideal for manual operation or as part of an automated workflow.
We also provide applicators for bottles and plates, including the Hermes Q – for both bottles and larger cylindrical containers. We can customize these by building bespoke ‘nests’ to suit specific container sizes and multiple nests to hold different containers.
Custom engineering for fully automated sample processing
For us, engineering a custom solution always starts the same way, with a thorough exploration of the customer’s needs and goals. When the definition is clearly established and agreed on, the iteration begins.
We build from scratch to integrate all the necessary components that achieve precisely the functionalities you require. This could involve bulk feeds, conveyor systems, robotics, and more.
The video shows just such a custom build for pre-barcoded tube production in action.
Automation is not always a big-ticket item
Today, in-house automated systems can be surprisingly affordable to create. ‘Off-the-shelf’ machines such as Germany’s cab AXON units are designed for flexibility and adaptability, and the wider adoption of automation across the world has made it far more accessible in terms of cost.
If in-house automation just doesn’t add-up for your organization, Computype can take on the investment for you, because we use many of the same applicators we supply to our customers.
A further option is our pre-barcoded service. We can perform the whole labeling process for you to your precise specifications. Computype can also handle other preparation tasks such as tare weighing and kitting. For many labs, the ability to release skilled people from these time-consuming ‘housekeeping’ activities offers attractive productivity benefits.
Even when in-house automation is not an organization’s primary strategy, it can perform a useful supplemental role. It can serve to perform ‘emergency’ labeling, or satisfy a need for reactive label delivery at low volumes.

Start tracking your success today.
Contact us now if you would like to learn more about Computype’s preparation products and services – our experts would be pleased to talk through all your options.
Schedule your Free Consultation
Pre-Barcoded Labware Services
The pre-barcoded, prepared labware options
Why consider the idea of outsourcing labware preparation? Going back to basics, it’s worth remembering that preparation tasks aren’t science. Most staff in a lab or life sciences process facility are skilled scientists or engineers, and some are highly skilled. So why pay people to do tedious work that’s beneath their expertise, that’s not their core scientific role, and not really contributing to the overarching scientific mission of the lab?
At Computype we’re happy to specialize in preparation. We’ve invested a lot of years, experience, and resources in perfecting the supply of pre-barcoded and prepared labware. The result is a service that’s tried and trusted for accuracy, quality and reliability.
It’s the go-to option for supporting biological inventory systems, compound library management, high-throughput screening, bio repository sample management, blood-related processes and more. Because where high value and safety-critical assets are concerned, only a highly evolved and thoroughly proven system will suffice.
Here’s an overview of what we do and why it makes scientific sense for our users…

Sequence management
We’re adept at achieving secure sequencing customized to your numbering convention and ensuring no duplicates. Errors and the time and expense of correcting them are reduced, and compatibility with your systems is guaranteed. This relieves you from the time and effort required for self-managed sequencing and reduces the risk of costly duplication errors. Preserving sequence integrity ensures we can employ your data to support studies and customer-specific data marking needs.
Labware procurement
Save time, effort, and money using Computype – and our buying power – to source your labware. Get the ideal labware for your work effortlessly. Gain from the simplicity of receiving the pre-labeled and prepared labware you need to be delivered directly to your facility
Tare weighing
Benefit from having your labware pre-weighed, cataloged, and ready to use as soon as it arrives on site. Each item weighed to a tenth of a milligram accuracy – +/- 0.2 or 0.5mg depending on the label material. Staff are free to work on their productive core activities.
Nase-controlled production
Be confident your labware has been unpacked, marked, and re-packaged in a Nase-controlled environment. Your pre-labeled labware is ready to use when DNA or RNA contamination must be avoided. Staff can concentrate on their higher-value core tasks.
Sorting, kitting, and packaging
Computype performs capping or de-capping, plugging, racking, and sorting tasks. It avoids investing in automated handling equipment or outsourcing to another vendor. Staff are free to work in their primary roles.
Labware and kits assembled to your bespoke specifications, including brand standards. Saves the time, space, and expense of packaging in-house. Assures on-brand presentation to end users
Kitting performed to your specific needs. Staff is freed to perform other tasks, and space becomes available for other uses. Labware is immediately ready to use on delivery.

Optimize data integrity
With expertly printed and applied barcodes and sequential order management, the potential for human error can be virtually eliminated. Further, advanced marking technologies and barcodes explicitly engineered to withstand demanding lab processes allow samples to remain consistent and legible over time.
Waste caused by unidentifiable samples and manual re-labeling tasks is also eradicated.
When the decision is made to partner with an identification and tracking team of experts, the container marking process, data integrity associated with each sample is drastically improved. Sequence management can now be assumed through your chosen partner to eliminate the possibility of duplicate codes in addition to issuing order releases based on specified barcode ranges.
This can be especially beneficial for libraries with compounds in multiple sites or libraries that have merged or shared compounds for expanded research. In addition to barcode data integrity, outsourcing the pretaring of containers also provides increased data control as an output file can be provided to cross-reference each code with its associated tare weight.
Importing/merging this data with your Laboratory Information Management System (LIMS) will ensure a strong and consistent foundation before involving intangible compounds or irreplaceable biologics

Freeing skilled staff for more productive work
Without the need to procure, label, and pre-tare labware, scientific staff can focus their time on the productive, skilled work their experience and education have equipped them for. As a result, productivity and job satisfaction are enhanced, and staff is freed from the risk of developing repetitive strain injuries.
Over time, this will not only improve consistency in sample tracking, but the quality of the research itself will continue to be realized through better utilization of the researcher’s time and tighter controls of basic quality processes.
In cases where sample libraries have been merged or shared, both parties can rest assured that, since entrusted to a single supplier, any relabeling or re-classification will result in samples that are accurately tracked and traced throughout the research and utilization process.
Entrusting non-value-added tasks to a supportive partner means the in-house benefits of time savings and reallocation of resources will be realized in terms of value-added research and a more reliable library of samples.
Achieve lean manufacturing in the lab
As lean manufacturing processes and 5S events become more and more popular in lab settings, eliminating unnecessarily stocked supplies and increased lab space can assist in reaching more strategic organizational goals related to quality and throughput.
Defects
Pre-barcoded labware ensures defects and waste is minimized as each container has been inspected for quality and is guaranteed to have a unique identifier that will withstand the specified storage conditions and environmental hazards. Following a strict quality approach at this preprocessing stage ensures that corresponding steps in the workflow aren’t compromised due to illegible traceability of a compound or biologic.
Over-Production and Inventory
Laboratories are often harsh environments, exposing samples and containers to chemicals, temperatures, abrasion, and other necessary procedures. Barcodes need to survive that environment without fading, failing, or falling off. Also offered as a component to outsourcing services can be that of inventory management programs.
Through utilizing such services, you’ll only have the labware on hand that you deem necessary for immediate/near future use, thus reducing space needed to house unneeded inventory. Furthermore, inventory related to labels, media, and labware is virtually eliminated, hence freeing up space for improved organization.
Transportation, Motion, and Waiting
When receiving labware that’s ready to use, several processes can be removed entirely froah for weighing, or then put into storage until time of use. Through utilizing a service for these activities, internal transportation related to labeling processes are often reduced by up to 75%. Furthermore, pre-labeled and tared labware can be batched and shipped to the site of intended usage based on demand schedules to even further reduce processing and handling.
Receiving labware that’s already labeled and tared equates to fewer steps in the prepping process, and hence, fewer opportunities for wasted downtime. Additionally, when barcode sequence management services and tare weigh output files are provided, there is less time needed to process data associated with the identity/ start weight of a container.

Preparing for a strong audit trail
Another significant limitation of manual processing concerning samples is data integrity and chain of custody. When merging a sample base and manually identifying samples, the possibilities for enhancing data integrity and maintaining a solid chain of control are more challenging. Legal imperatives are driving the need for a clear audit trail that directly correlates to the unique information stored on each sample’s barcode identifier, particularly concerning hazardous, therapeutic, or controlled substances. Equally, the high value/cost of specific compounds or samples means organizations can ill afford to employ a less-than-robust tracking and barcoding system.
How pre-barcoded labware reduced labor and streamlined operations for a large cell and DNA repository
One of the most considerable cell and DNA repositories in the world’s primary focus is supporting scientific research in finding cures for complex genetic diseases. Receiving samples from over 500 domestic and international collection sites, they also supply transformed human cell lines, DNA, RNA, and other biomaterials to researchers targeting genetic causes for disease management. Over the last ten years, this repository has produced over 200,000 cell lines and distributed more than 600,000 DNA samples and biomaterials to National Institute of Health-approved investigators.
This company had been struggling with inefficiencies in its tracking and labeling processes and reached out to Computype hoping to find a solution. This customer had been previously tracking its samples with manually applied linear barcodes. In addition to the inefficiencies, cost was a primary concern. Many of their contracts are
based on government bids awarded in multiyear terms and with established fixed costs, so it was necessary to seek an affordable solution, so it was necessary to seek an affordable solution.
After fully understanding their concerns and learning about their business processes, Computype proposed our Pre-Barcoded Labware Services program. With this service, we were able to provide the customer regular shipments of their specifiepressure-sensitived labware pre-barcoded with Direct Mark technology to boost efficiencies and eliminate manual labeling from the researchers’ jobs. This was a gleaming success; not only did this solution put an end to manually applying pressure sensitive labels in-house, but the use of a Direct Mark 2D code on the container
took up much less surface space, while still ensuring consistent and reliable identification through the workflow.
The second component of the solution came from Computype’s partnership with glassware manufacturers. Because of this strong working relationship, we were able to directly procure the needed labware, ultimately saving the repository shipping expenses and paperwork, while decreasing turnaround times and streamlining the process. The customer’s willingness to modify their processes and place their trust in Computype enabled a solution that would most benefit their strategic goals.
In the less than six months from conception to implementation, the repository was receiving regular shipments of pre-marked vials packed in sequential order for ease of use. This solution was completed on time, within budget, and integrated seamlessly into their current lab operation.
Can pre-barcoded, prepared labware reduce friction in your lab?
Preparatory tasks often cause friction in delays within laboratory workflows. Speak with an expert today to learn how you can receive expertly prepared labware ready to use.
5. Digital traceability: Track, inform, predict.
In an increasingly complex landscape of evolving technologies, globalization and data proliferation, simply keeping track of assets such as samples or specimens can often be a major challenge.
For those managing laboratories and related facilities, the opportunities offered by technology strands such as machine learning, AI, VR, AR, and others are often tempered by systemic incompatibilities. Data will often end up existing in effectively separate ‘islands’. Mergers, acquisitions or collaborations can mean different ways of handling workflows and data must somehow be unified…
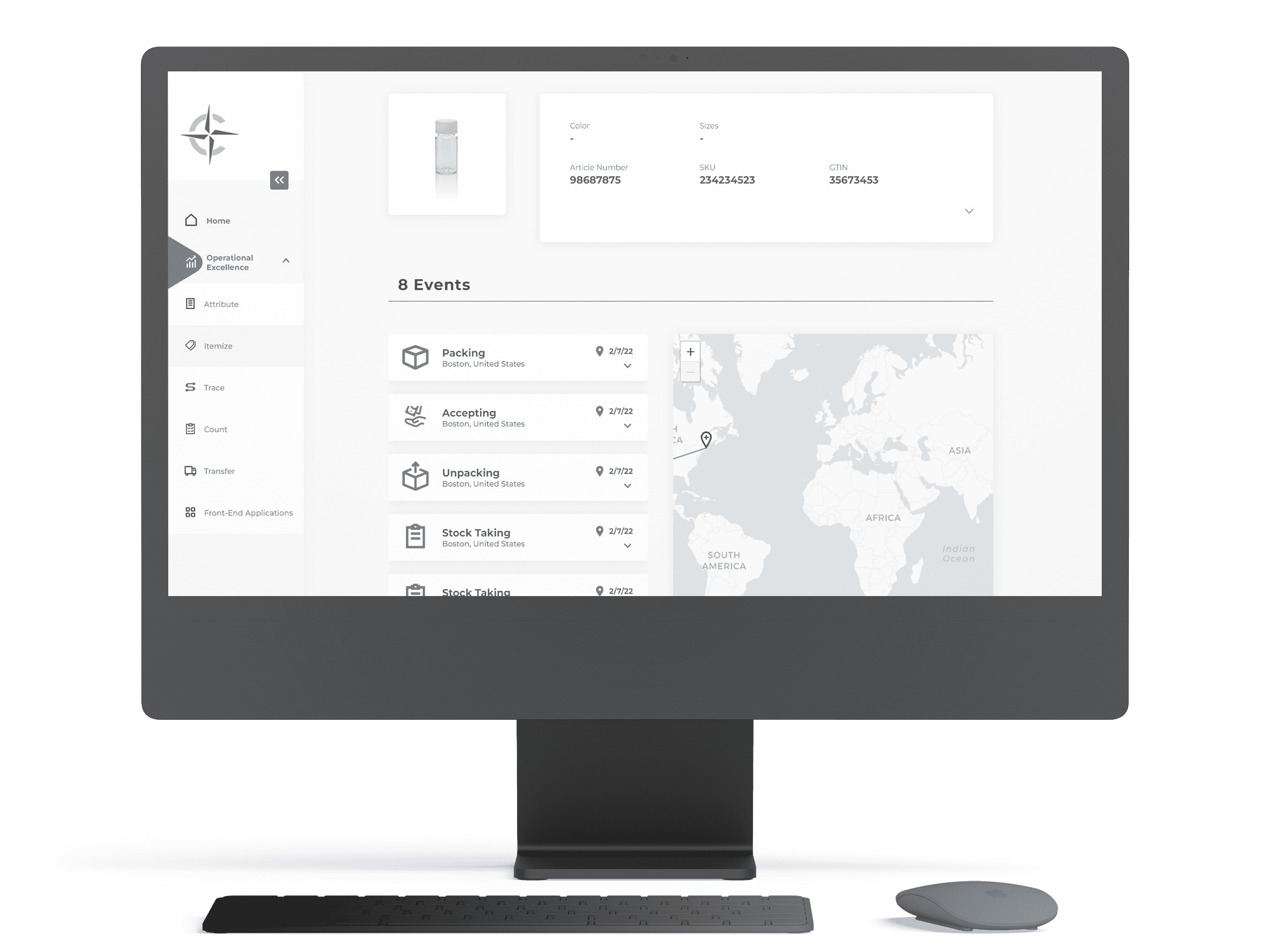
Whatever the combination of issues, Computype’s Digital Traceability Platform (DTP) can help provide the tracking and traceability for end-to-end visibility of your organization’s assets.
Making the connections
To take the example of our natural world, the astonishing interconnections and interdependencies of the environment are what hold the whole together. Our world of data is similarly immense, but where there’s a lack of interconnection, things can get dysfunctional.
At Computype we don’t claim to have all the answers for all the world’s data management woes. But we do have the means to straighten things out when it comes to identifying and tracing assets, particularly in the realm of laboratories and related facilities.
Where there are different systems such as laboratory information management and warehouse management, where assets transit through facilities and disparate work modes, Computype DTP makes sure it’s all connected, it fills in the gaps.
Compounds and biologics
Today’s compound libraries are increasingly expanding to include biologics; accommodating both kinds of assets inevitably creates challenges. These two disciplines can have distinctly different processes, demanding storage requirements, different workflows. These factors will often mean that disparate tracking and tracing methods must now be brought together – this is the kind of demanding task that Computype DTP has been designed and built for.
Pooling resources
Businesses and organizations are increasingly coming together in different ways for different reasons. Mergers, acquisitions, partnerships, one-off collaborative projects – whatever the catalyst, the pooling of resources and expertise can be a powerful tool for achieving specific aims.
There’s a strong trend in the pharmaceutical, biotech, and academic worlds towards collaboration and the sharing of sample libraries. The advantages of increasing a sample base effectively overnight can be transformational, just so long as all the libraries, processes, and tracking systems involved can be successfully integrated. Achieving that state of connectedness takes experience twinned with a strong digital traceability platform.
Data exchange and integration
Receive and share asset-specific data from and to virtually any other data system in a customer’s operation as well as up- or downstream all via APIs. Tie together multiple systems such as LIMS or Lab Notebooks.
- Laboratory Information Management Systems (LIMS)
- Electronic Lab Notebooks
- Instrument Data Systems
- Warehouse Management Systems
- Enterprise Resource Planning
Digital traceability platform
The Digital Traceability Platform (DTP) enhances and enables scientific processes and the delivery of laboratory information. This solution creates an overlay connecting existing digital systems (LIMS, Warehouse Management, Enterprise Resource Planning, etc.) to provide a centralized data hub. Many laboratories must pull information from several sources when moving assets through their workflows and reporting on fundamental metrics. The Digital Traceability Platform would allow laboratory staff to access all of their information through one interface.
Centralized hub of data
Customer-defined dashboard widgets display at-a-glance metrics and status updates for assets Customer-defined dashboard widgets display at-a-glance
Immutable chain of custody
All status and location updates are recorded. No changes are erased for reliable chain of custody history and worry-free audits.
Callouts
Callouts highlight events which require attention. Callouts may indicate invalid item states, expiry of certificates, stock outages or whatever is germane to your activities.
Machine learning
DTP applies an ensemble of different heuristics and machine learning algorithms to identify patterns and predict potential outcomes.
Powered by the atma.io connected product cloud by Avery Dennison
Recently, we’ve entered a partnership with atma.io to bring our customers the ability to track the digital life of key medical assets through the new Computype Digital Traceability Platform.
The platform is powered by atma.io, the connected product cloud by Avery Dennison which unlocks the power of connected products by assigning unique digital IDs to everyday items. This is already used to manage 22 billion items by organizations across the apparel, retail, food, and life sciences industries.
Max Winograd, co-founder atma.io and vice president, connected products, Avery Dennison Smartrac adds: “Since we launched atma.io, interest in the platform has been phenomenal with wide appeal across a variety of segments. We now manage billions of items, many of which are perishable, life-or-death products, and need to be kept under precise conditions such as vaccine vials.
Our partnership with Computype and the introduction of their Digital Traceability Platform powered by atma.io will help numerous companies in the biopharmaceutical and life sciences segments reduce waste, improve product safety and make more sustainable choices. We’re excited for this powerful combination of a proven, world leading connected product cloud and an industry leader in comprehensive identification solutions.”
Opening silos, gaining insight
Over time data can wind-up in isolated silos – maybe as a result of legacy systems being incompatible with newer solutions, departmental separations, organizational restructuring, or a history of piecemeal procurement. Whatever the reasons, it’s frustrating to know that while there’s useful data in there, it’s just not possible to utilize its full potential.
With Computype’s expertise in unifying different systems via its Digital Traceability Platform, all data sources can be integrated for smooth workflows and reporting that can directly inform business decisions.
Enterprise reporting and predictive analytics
With data collection comes data-driven results and insights. This track and trace monitoring solution integrates into your existing systems to provide intelligence that can drive analytic decisions and process improvements. Generate powerful analytics reporting using Microsoft PowerBI or leverage data for your own analytics platforms. View integrated analytics such as most active locations and common issues or events.